Compare And Contrast Nuclear Fusion And Nuclear Fission.: Complete Guide & Key Details
Alright folks, let's talk about atoms. Specifically, those tiny, powerful little things that make up everything. We're going to peek behind the curtain at two atomic superstars: Nuclear Fission and Nuclear Fusion. Now, you might hear these words and think, "Oh no, science class flashbacks!" But trust me, this is going to be more like a fun, slightly explosive family reunion.
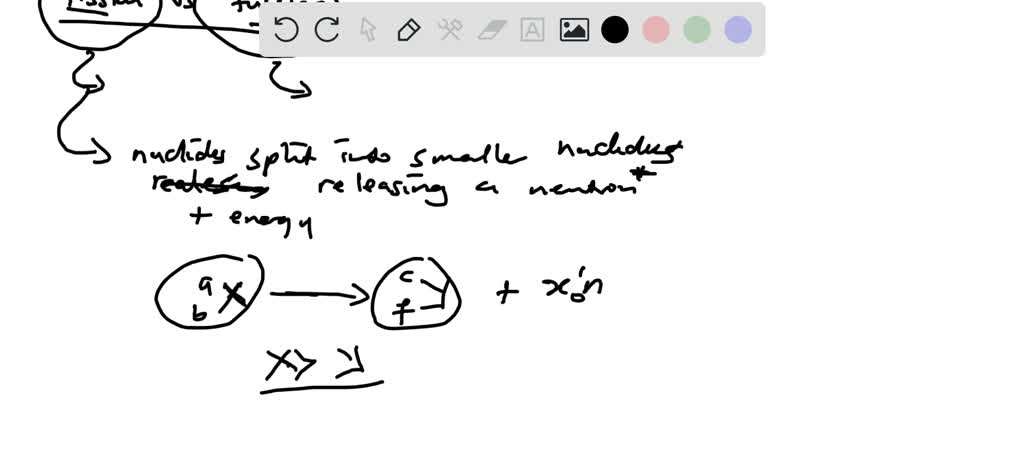
Think of it this way: Nuclear Fission is like a giant, overstuffed piñata. You give it a good whack, and BAM! It breaks open, scattering all sorts of smaller bits and pieces. In the atomic world, this means taking a really big atom, like Uranium, and splitting it into two smaller atoms. This splitting act releases a ton of energy. It's a bit messy, a bit dramatic, and definitely creates a whole lot of stuff.
We've been using fission for a while now. It powers our nuclear plants, which are like the hardworking grown-ups of the energy world. They get the job done, churning out electricity. But, and this is where it gets a little less rosy, fission also leaves behind some not-so-great leftovers. We call these radioactive waste. It's like the glitter from the piñata that gets everywhere and is super hard to clean up. It stays grumpy and dangerous for a very, very long time. So, while fission is a reliable power source, it's also a bit of a high-maintenance guest.
Now, let's switch gears to Nuclear Fusion. This is where things get truly exciting. Imagine instead of breaking things apart, you're bringing things together. Fusion is like two tiny, energetic toddlers who are so excited to play they decide to hug really, really hard. When they hug, they become one slightly bigger toddler, and poof! A massive burst of energy is released. This is what happens in stars, like our very own Sun. The Sun isn't just a big ball of fire; it's a giant fusion reactor!
The fuel for fusion? Tiny atoms like Hydrogen. Super abundant stuff, unlike the precious Uranium we need for fission. And here's the really good part: when fusion happens, the "leftovers" are mostly harmless. We're talking about things like helium, the stuff that makes balloons float. No long-term, grumpy waste to worry about. It's like the toddlers just high-five and run off to play more.

So, what's the catch with fusion? Why aren't we all running on star power from our backyards? Well, creating those super-exciting toddler hugs (or atomic collisions) takes an unbelievable amount of heat and pressure. We're talking hotter than the core of the Sun. Trying to replicate those conditions here on Earth is like trying to build a tiny sun in your living room. It's incredibly difficult and expensive. Scientists are working on it, bless their determined hearts, but it's a tough nut to crack. Think of it as the incredibly talented artist who just hasn't quite finished their masterpiece yet.
Here's a quick rundown, just to make it crystal clear:

- Fission: Breaking big atoms apart. Think of it as a controlled explosion.
- Fusion: Smashing small atoms together. Think of it as a super-powered hug.
Fission is our current, reliable, but slightly messy energy source. It's like the reliable old car that gets you where you need to go, even if it coughs a bit and needs frequent oil changes. Fusion, on the other hand, is the dream car. It's super clean, incredibly powerful, and the ultimate goal, but it's still in the concept and prototype stage. We're all eagerly waiting for it to hit the market.
My unpopular opinion? I kind of love fusion. It just feels so... optimistic. It's the universe showing us what pure, unadulterated power can do when it's harnessed cleanly. It’s the promise of a future where we have all the energy we need without all the messy baggage. While fission has served us well, and will continue to for a while, my heart (and my inner science nerd) belongs to the stars and the incredible dance of fusion. It's the ultimate cosmic party trick, and I, for one, can't wait to see it at full swing.
