Please Predict The Products For Each Of The Following Reactions:: Complete Guide & Key Details
Ever wondered what happens when you mix different ingredients together? In the world of chemistry, it's like a magical culinary show! We're talking about predicting the products of various reactions. It’s a bit like being a detective, but instead of solving crimes, we're figuring out what amazing new things will be created.
Think of it as a puzzle. You’re given a starting set of ingredients, and with a little bit of know-how, you can guess what the final dish will be. It's not about memorizing a million recipes, but understanding the basic flavors and how they combine. This makes chemistry so much fun, because it’s all about discovery!
So, what makes this "predicting the products" stuff so cool? It’s the feeling of being a chemist, even if just for a moment. You get to look at a reaction and say, "Aha! I bet this will turn into that!" It’s about logic, patterns, and a touch of educated guessing.
It’s like playing a game where the rules are set by nature, and you’re learning to play them well. The more you understand these rules, the better you get at predicting what will happen. And that’s incredibly satisfying!
Let's dive into some examples. Imagine we have hydrogen gas (that's H₂) and oxygen gas (that's O₂). What do you think they make when they get together, maybe with a little spark? If you guessed water (H₂O), you’re spot on! It’s a classic reaction, and predicting it is the first step to understanding so much more.
This is where the magic begins. We see the reactants, which are our starting ingredients. Then, we use our knowledge to figure out the products, the brand-new substances that are formed. It’s like looking at LEGO bricks and knowing exactly what you can build with them.
There are certain types of reactions that happen over and over again. Recognizing these patterns is the key to becoming a product prediction pro. It’s not about brute force memorization; it’s about understanding the underlying principles.
One common type is a synthesis reaction. This is where two or more simple substances combine to form a more complex one. Think of it as building something bigger from smaller pieces. Like combining letters to make a word.
Another is a decomposition reaction. This is the opposite of synthesis. A complex substance breaks down into simpler substances. Imagine a complex cake crumbling into its basic ingredients: flour, sugar, and eggs.
Then we have single displacement reactions. Here, an element from a compound is replaced by another element. It’s like a dance where one partner steps out and another steps in. One substance gets "displaced."
And let’s not forget double displacement reactions. In this case, the positive and negative ions of two ionic compounds swap partners. It’s a bit like a double-date where everyone switches partners.
Predicting products is not just about writing down chemical formulas. It’s about understanding the forces that hold atoms and molecules together, and what makes them want to break apart and reform. It's a peek into the hidden world of chemical transformations.
The truly special thing about this is its predictive power. Instead of just observing what happens, you can anticipate it. This allows scientists to design experiments and create new materials. It’s a fundamental skill for anyone exploring chemistry.
Let’s look at another example: sodium (Na) reacting with chlorine (Cl₂). These are both quite reactive elements. When they combine, they form sodium chloride (NaCl), which is just plain old table salt! See? Everyday stuff can come from these reactions.
The key to predicting these is often looking at the types of elements involved. Are they metals? Nonmetals? Do they tend to lose or gain electrons? These clues help us figure out the final arrangement.
For instance, Group 1 metals like sodium are very eager to lose an electron. Halogens like chlorine are very eager to gain one. When they meet, it's a perfect match, and they form a stable compound.
Consider a reaction like the burning of methane (CH₄) in oxygen (O₂). This is a combustion reaction. When hydrocarbons burn completely, they typically produce carbon dioxide (CO₂) and water (H₂O). So, CH₄ + O₂ will yield CO₂ + H₂O. It's a predictable outcome for this type of reaction.
What makes this entertaining is the element of surprise, even when you're predicting. Sometimes the products can be unexpected, or there might be different pathways a reaction can take. It keeps you on your toes!
Imagine being handed a recipe for a cake, but instead of knowing the final cake, you only know the ingredients. Predicting the products is like figuring out whether it will be a chocolate cake, a vanilla cake, or maybe even a fruitcake!
It’s not just about what can form, but also what is most likely to form under given conditions. Factors like temperature and pressure can influence the outcome, adding another layer of intrigue to the prediction.
Let’s consider potassium (K) and water (H₂O). Potassium is a very reactive alkali metal. When it reacts with water, it produces potassium hydroxide (KOH) and hydrogen gas (H₂). It's a vigorous reaction, and predicting it involves understanding potassium's reactivity.
This is where understanding activity series comes in handy. It’s a list that ranks elements based on their reactivity. If a more reactive element is present, it can displace a less reactive one in a compound.
For example, if we have a solution of copper sulfate (CuSO₄) and we add a piece of zinc (Zn), what happens? Zinc is more reactive than copper. So, the zinc will displace the copper, forming zinc sulfate (ZnSO₄) and solid copper (Cu). It's a visual transformation you can often observe.
The beauty of predicting products is that it builds upon itself. As you learn about one type of reaction, it helps you understand others. It’s like learning new vocabulary words; they help you construct more complex sentences.
What’s truly special is when you can predict a reaction that leads to something useful. Like predicting the products of a reaction that creates a new medicine or a material for building something amazing. The practical applications are vast.
Let’s take another look at double displacement. If we mix silver nitrate (AgNO₃) with sodium chloride (NaCl), what do we get? They swap partners! We get silver chloride (AgCl) and sodium nitrate (NaNO₃). The cool part is that silver chloride is an insoluble solid, meaning it forms a precipitate – a visible clue that the reaction happened!
This is another element of fun: sometimes the products tell you they are there! Precipitates, gas bubbles, or heat changes are all indicators. Predicting them means you know to look out for these signs.
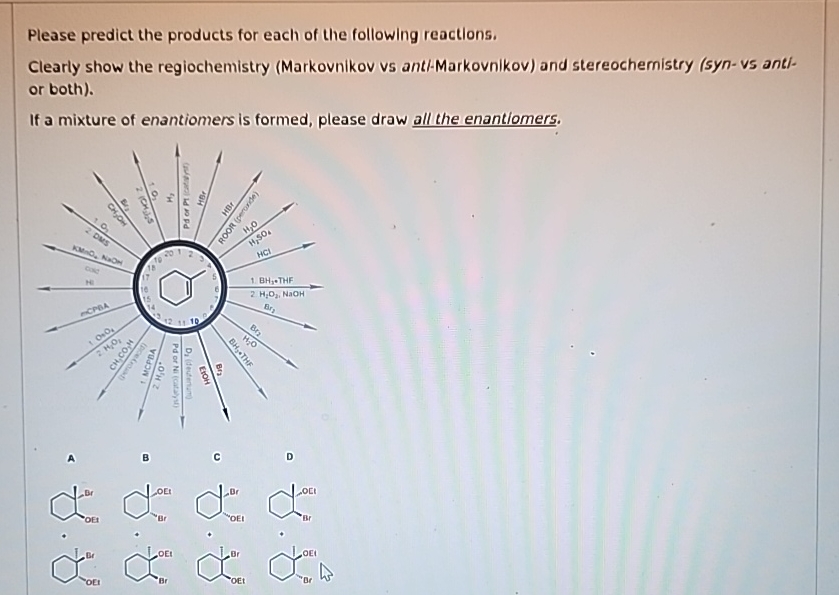
Predicting products also requires understanding ions. These are charged atoms or molecules. They are the tiny building blocks that often do the swapping and combining. Knowing their charges is crucial.
For example, in silver nitrate, we have Ag⁺ and NO₃⁻. In sodium chloride, we have Na⁺ and Cl⁻. When they mix, Ag⁺ pairs with Cl⁻ to form AgCl, and Na⁺ pairs with NO₃⁻ to form NaNO₃. The charges must balance for a neutral compound.
This journey of predicting products is filled with lightbulb moments. It’s where abstract chemical concepts start to make real-world sense. You begin to see the world around you in a new chemical light.
It’s engaging because it taps into our natural curiosity. We see things react all the time – from baking a cake to rust forming on metal. Chemistry helps us understand the "why" and "how" behind these transformations.
The challenge lies in mastering the various reaction types and the rules that govern them. But with each correct prediction, there’s a sense of accomplishment and a deeper understanding. It’s a rewarding process.
So, the next time you see two substances together, let your inner chemist take over! Try to predict what they might create. It’s a fantastic way to explore the fascinating world of chemical reactions and discover the amazing possibilities that lie within. It's a constant adventure!
